16.8: Application of HLA testing to patient and donor investigations
16.8.1: Investigation of refractoriness
Please refer to the relevant EFI Standards for Transfusion.
The most common cause of immunological refractoriness to random donor platelet transfusion is the presence of HLA-specific antibodies in the patient receiving platelet transfusions. The management of this group of patients may involve the provision of HLA-compatible platelets
HLA Class I typed platelets should normally be provided for refractory patients with the aim of minimising exposure to mismatched Class I antigens. In the absence of a zero mismatched donor, a compatible donor can be selected on the basis of a lack of antigens or alleles corresponding to the antibody specificities identified in the patient.
The investigation of refractoriness (see Figure 16.1) and the provision of selected platelets in such cases should comply with the British Committee for Standards in Haematology (BCSH) Guidelines for the Use of Platelet Transfusions5. Serological investigation of suspected immune refractoriness requires screening for HLA Class I-specific antibodies only, but the screening technique must detect HLA-A, HLA-B, and HLA-C-specific antibodies. Any screen-positive patient should be tested further for specificity to include all the Class I antigens listed in Table 16.2.
If a patient has HLA-specific antibodies that cannot be completely characterised, or a specificity corresponding to any of the donor’s HLA Class I antigens cannot be excluded, then a crossmatch between donor and patient may be performed as described above.
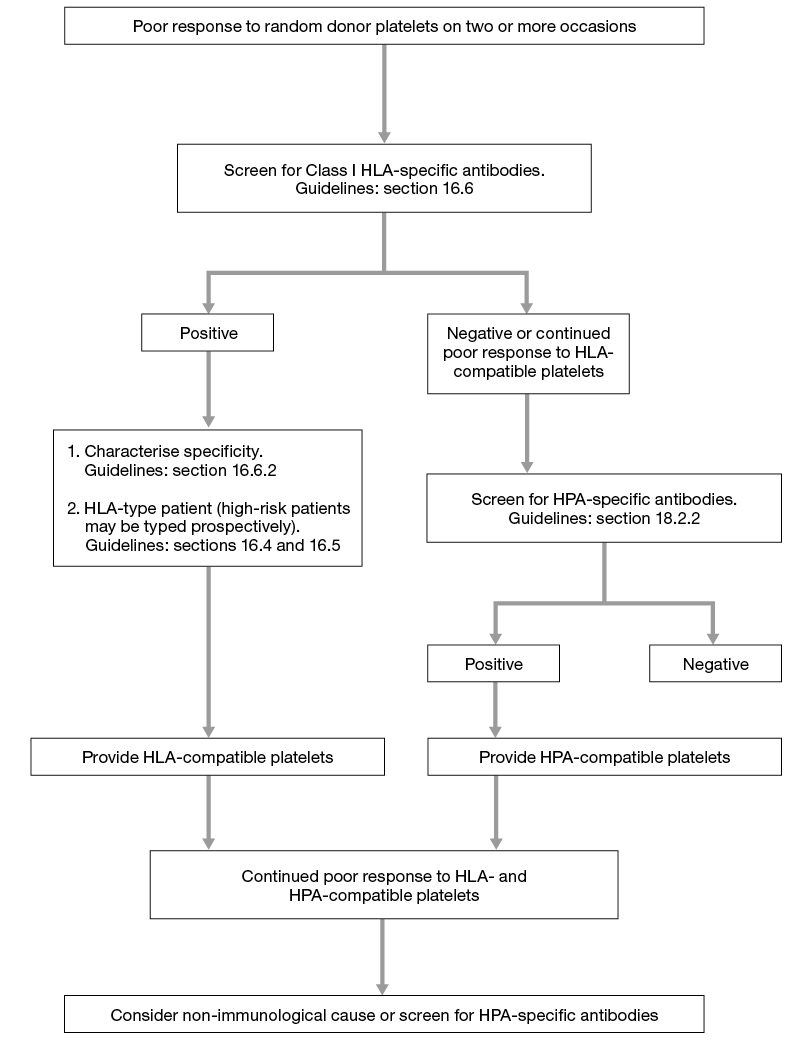
Figure 16.1 Algorithm for laboratory investigation of platelet refractoriness
16.8.2: Investigation of TRALI
Please refer to the relevant EFI Standards for Transfusion.
HLA and/or granulocyte-specific antibodies present in donor plasma have been implicated in nearly 80% of TRALI cases (patient leucocyte antibody or inter-donor reactions in pooled products have also been reported as causes of TRALI). The identification of leucocyte-specific antibodies in implicated donors provides support for the diagnosis of TRALI.
Sera from all implicated donors must be screened for both HLA Class I and Class II specific antibodies and HNA antibodies (see section 16.6.2). Any screen positive serum should be further characterised for HLA Class I and Class II to identify the antibody specificity
If any of the implicated donors are shown to have HLA-specific antibodies the patient should be typed for HLA Class I and Class II to determine the presence of alleles/antigens corresponding to the antibody specificities found in the donor(s).
If a donor serum has HLA-specific antibodies that cannot be completely characterised, or a specificity corresponding to any of the patient’s HLA antigens cannot be excluded, then a crossmatch between donor and patient should be performed.
See Figure 16.2 which gives an algorithm for laboratory investigation of TRALI.
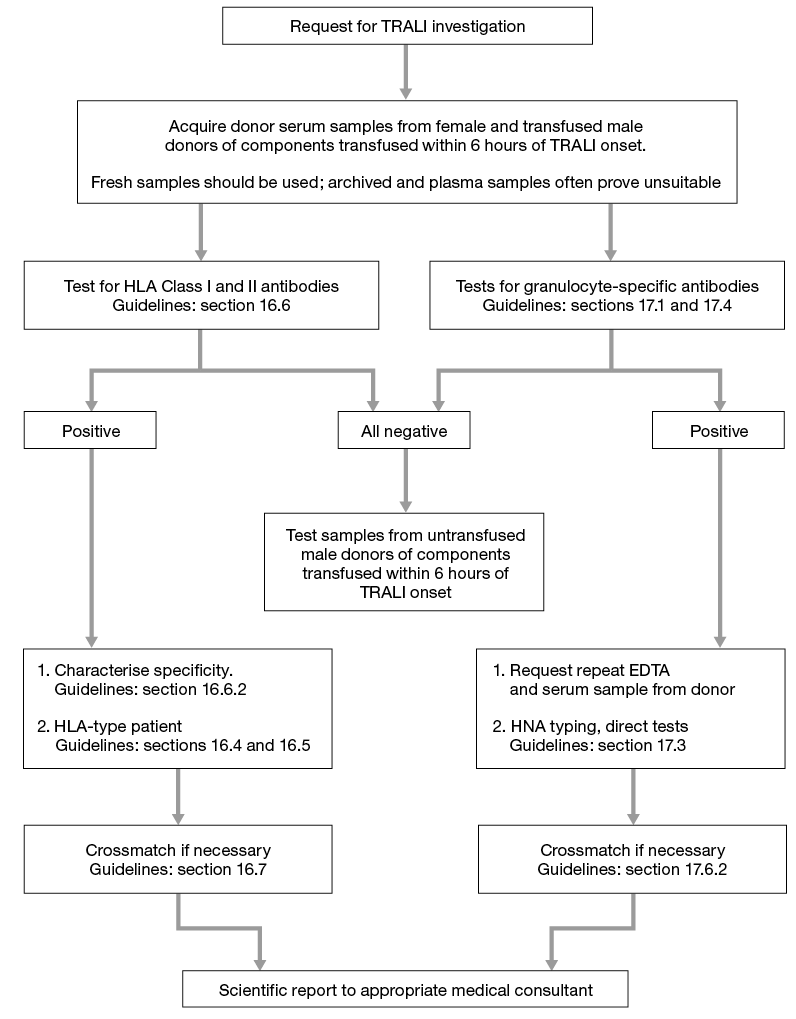
Figure 16.2 Algorithm for laboratory investigation and reporting of TRALI case
16.8.3: Investigation of febrile transfusion reactions
Please refer to the relevant EFI Standards for Transfusion.
If an investigation is requested, sera from patients should be screened for both HLA Class I and Class II-specific antibodies. Any screen-positive serum should be further characterised for HLA Class I and Class II specificities to include all those listed in Table 16.2.
16.8.4: Apheresis platelet donors
Please refer to the relevant EFI Standards for Transfusion.
All potential apheresis platelet donors used for the provision of HLA selected platelets should be typed for HLA-A, HLA-B and HLA-C.
DNA-based typing should allow for HLA alleles to be defined to at least the two-digit (first field) level of resolution.
Each donor should be HLA typed twice using samples collected on separate occasions, such that only if the second test confirms the first should the donor provide platelets for clinical use.
16.8.5: Testing of donors/cord units for related haematopoietic stem cell transplant
Please refer to relevant EFI standards for Haematopoietic Stem Cell Transplantation.
DNA-based HLA typing, to at least the two-digit (first field) level of resolution, should be performed on donors. High-resolution typing may also be necessary as detailed below.
Initially, all potential related stem cell donors must be typed for at least HLA-A, HLA-B and/or HLA-DR to assess compatibility. Further testing must then be undertaken to establish a phenotypic match for HLA Class I and II loci, as described in local protocols. HLA types of the matched patient and donor must be confirmed on a second sample. If HLA haplotype inheritance can be established by typing family members, then high-resolution typing is not required to establish a genotypic match. However, if haplotype inheritance is not established, high-resolution typing of HLA Class I and/or Class II should be undertaken as required by the local transplant protocol. Intra-familial donors who are not HLA identical siblings require both Class I and Class II high-resolution typing as required by the local transplant protocol.
As a minimum related cord units must be typed at low resolution for HLA-A, -B and -DRB1. Extended typing must be undertaken if required by the transplant protocol.
Prior to cord unit transplant, confirmatory typing at low resolution must be performed for HLA-A, -B and -DRB1. Typing must be performed on a segment of the tubing integrally attached to the unit, on a satellite vial or on the content of the thawed unit.
16.8.6: Testing of donors/cord units for unrelated haematopoietic stem cell transplant
Please refer to relevant EFI standards for Haematopoietic Stem Cell Transplantation.
DNA-based HLA typing, to at least the two-digit (first field) level of resolution, should be performed on donors. High-resolution typing may also be necessary as detailed below.
As a minimum all potential unrelated donors should be typed for HLA-A, -B , -C and -DRB1. HLA types of patient and donor should be confirmed, although the original type from the unrelated donor registry is acceptable for this purpose. The need for high-resolution typing of HLA Class I and II will depend upon local transplant protocols.
As a minimum cord units must be typed at low resolution for HLA-A and -B and high resolution for -DRB1. Extended typing must be performed if required by the local transplant protocol. Prior to commencement of patient conditioning, a minimum low-resolution confirmatory type of at least HLA-A, -B and -DRB1 must be performed upon receipt of the shipped unit. Typing must be performed on a segment of the tubing integrally attached to the unit, on a satellite vial or on the content of the thawed unit.
16.8.7: Investigation of female donors to reduce the incidence of TRALI
Many transfusion services screen for HLA or HLA and HNA antibodies to reduce the incidence of TRALI. An initial screen for HLA antibodies may be followed by a screen for HNA antibodies to further reduce the potential incidence of TRALI (see section 16.8.6). Female blood donors should be investigated for HLA antibodies following the guidelines set out in section 16.6. There is no requirement to determine the specificity of any HLA antibodies detected or type the donor for HLA.