1.1: Development of the Red Book
Guidelines for the Blood Transfusion Services in the United Kingdom was first published in 1990 by HMSO. It was compiled by experts from the then Regional Transfusion Centres and the National Institute for Biological Standards and Control (NIBSC), and aimed to define guidelines for all materials produced by the United Kingdom Blood Transfusion Services (UKBTS) for both therapeutic and diagnostic use. The driving force for this joint initiative, which started in 1987, was the imminent European Union (EU) Directive which would bind member states to introduce product liability by July 1988. It was understood that human blood and substances derived from it would be defined as ‘products’ in terms of this Directive, and guidelines against which manufacturers could be inspected would be required.
Since then seven editions of the Red Book (as the guidelines became known) have been published. They are compiled by a group of experts both from within the Blood Transfusion Services and from the wider NHS and universities, now called the Joint UKBTS/HPA Professional Advisory Committee (JPAC).
The Red Book contains guidelines reflecting best practice, sets standards to be met by the products, describes technical details of the processes involved and states the legally binding requirements introduced in 2005 under the Blood Safety and Quality Regulations, Statutory Instrument 2005 No. 50.1 More detailed information about the regulatory environment relating to blood and tissues in the UK can be found in Chapter 2.
Guidelines reflect best practice and are developed by professionals in the field. JPAC consists of such professionals in blood transfusion and tissue transplantation, appointed for their expertise from throughout the UK. The Red Book reflects their work as it is implemented in the UK. The book concentrates on the products rather than their use. Clinical use of blood and blood components is outlined in the Handbook of Transfusion Medicine,2 produced by collaboration between JPAC and the British Committee for Standards in Haematology (BCSH).
Professional guidelines are not legally binding but, as they reflect consensus best practice, may be taken into account by the UK judiciary. Such national guidelines have to take into account EU Directives, which have to be transposed into UK law, and these are legally binding.
JPAC, with its Standing Advisory Committees (SACs), undertakes regular review of the guidelines in the light of developments in the field, both scientific and regulatory. The overall aim is to ensure as far as possible the safety of blood transfusion in the UK, for both the donor and the patient. Changes in the guidelines are therefore likely to occur, and these will be notified on the JPAC website, www.transfusionguidelines.org.uk, where an up-to-date version of the guidelines can always be found.
The work of JPAC is conducted through expert SACs. The current SACs of JPAC are shown in Figure 1.1.
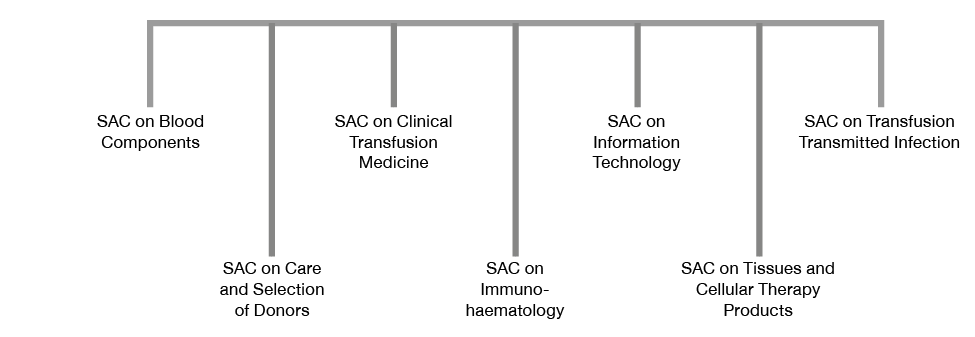
Figure 1.1 Standing Advisory Committees of the Joint UKBTS/HPA Professional Advisory Committee