Terms of Reference - April 2025
Download the Terms of Reference in PDF format
- 1. Introduction
- 2. Structure of JPAC
- 3. Terms of Reference
- 3.1. JPAC Board
- 3.2. Executive Working Group
- 3.3. Standing Advisory Committees
- 3.3.1. General to all SACs
- 3.3.1. SAC on Blood Components
- 3.3.2. SAC on Care and Selection of Donors
- 3.3.3. SAC on Cellular Therapy Products
- 3.3.4. SAC on Immunohaematology
- 3.3.5. SAC on Information Technology
- 3.3.6. SAC on Tissues
- 3.3.7. SAC on Transfusion Transmitted Infection
1. Introduction
1.1. Mission statement
The Joint Professional Advisory Committee to the UK Blood Transfusion and Tissue Transplantation Services (JPAC) has two distinct remits:
- To be an advisory committee to the UK Blood Transfusion and Tissue Transplantation Services.
- To prepare detailed guidelines for the UK Blood Transfusion and Tissue Transplantation Services.
JPAC guidelines, which constitute JPAC’s professional advice to the UK Blood Transfusion and Tissue Transplantation Services (UK Services), aim to be evidence based as far as possible and are reviewed regularly. The guidelines also take account of relevant Recommendations and Directives from European organisations such as the Council of Europe (CoE) and the European Union (EU). Services may choose to deviate from JPAC guidelines, but any such deviation should be substantiated and well documented.
1.2. Transparency and openness
JPAC operates from a presumption of transparency and in accordance with the requirements of relevant legislation, such as the UK General Data Protection Regulation (UK GDPR) and the Freedom of Information Act (FOIA).
All proceedings aim to maintain a high level of openness by clear documentation of decision-making processes and the timely publication of papers, minutes and other outputs from meetings where appropriate. The release of such documentation is guided by the requirements of the FOIA and supported by NHS Blood and Transplant (NHSBT), the host organisation for JPAC.
1.3. Declaration of interests
JPAC ensures that no conflict arises, or could be reasonably perceived to arise, between the public duties and private interests, financial or otherwise, of its members. All members of the JPAC Board, the EWG and the SACs are required to declare any interests upon appointment, and by annual declaration. Any new interests should also be declared at the next relevant meeting.
2. Structure of JPAC
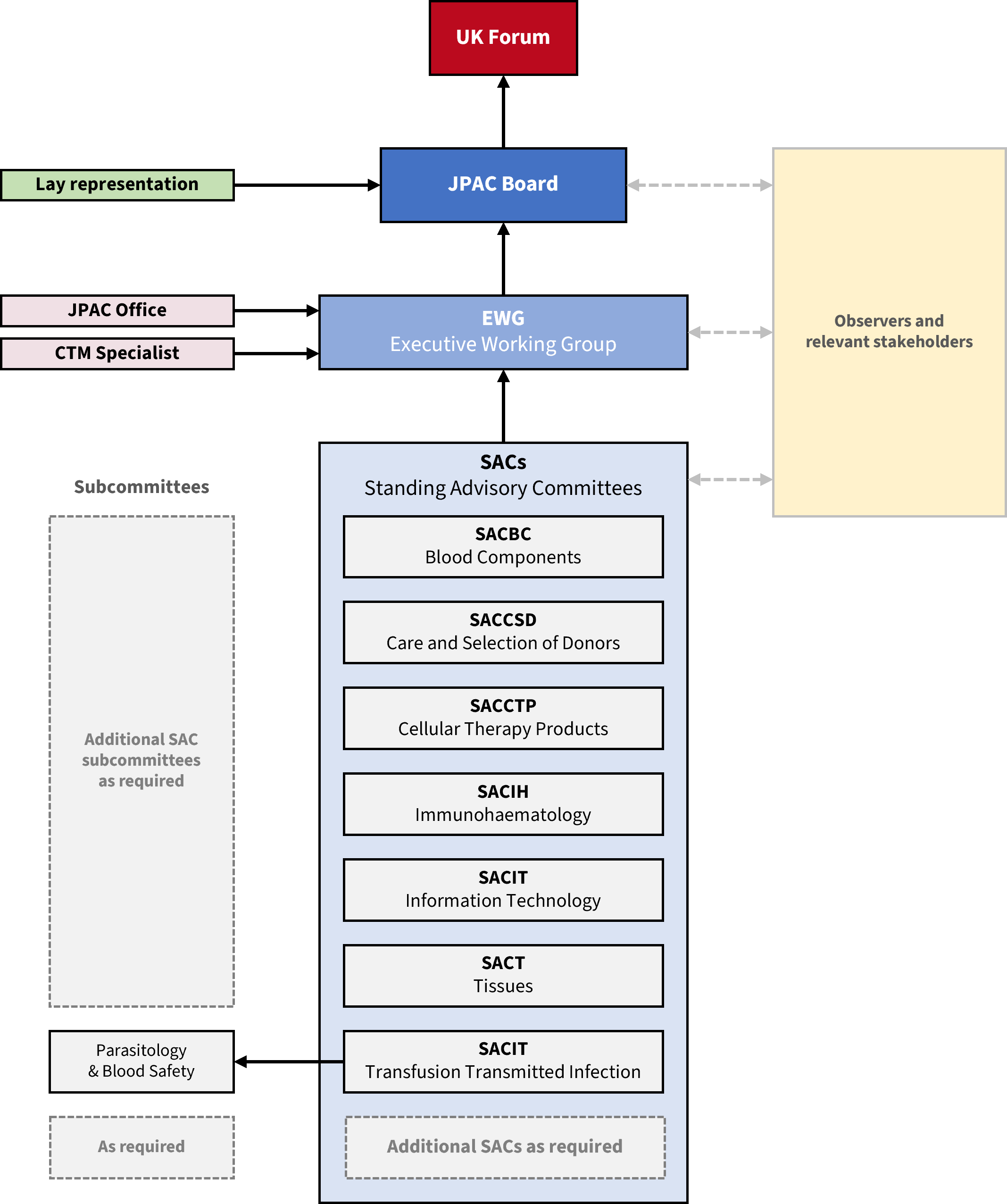
2.1. JPAC Office
The JPAC Office consists of the Professional Director, the Deputy Professional Director, the Scientific Lead for Safety Policy, the Scientific Publishing Manager, and the Administrator.
The JPAC Office reports to the EWG via the Professional Director.
2.2. Standing Advisory Committees
Each SAC consists of subject matter experts led by a Chair. The specific scope of each SAC is detailed in their own Terms of Reference, but a general remit applies to all. There are currently seven SACs and one SAC subcommittee. SACs, subcommittees and other ‘ad hoc’ working groups may be established and disbanded according to the needs of the UK Services.
All SACs report to the EWG via their Chair.
2.3. Executive Working Group
The EWG membership consists of the members of the JPAC Office, the Chairs of the SACs and a Clinical Transfusion Medicine (CTM) Specialist who acts as a formal clinical representative to JPAC.
The EWG meets six times per year and reports to the JPAC Board.
2.4. JPAC Board
The JPAC Board membership consists of the members of the EWG, the Medical Directors of the four UK Services, and representatives from relevant stakeholders including the Medicines and Healthcare products Regulatory Agency (MHRA), MHRA South Mimms Laboratories (formerly the National Institute for Biological Standards and Control, NIBSC), the Human Tissue Authority (HTA) and UK Quality Managers group (UKQM). The JPAC Board also includes permanent lay members as donor/patient/public representatives.
The Irish Blood Transfusion Service (IBTS) and Serious Hazards of Transfusion (SHOT) are invited observers. Additional ‘ad hoc’ observers may attend at the discretion of the Professional Director.
The JPAC Board meets three times per year and reports to the UK Forum.
3. Terms of Reference
Any changes to the overall remit of JPAC, or to the Terms of Reference for the JPAC Board or the EWG, must be approved by the UK Forum.
Any changes to the Terms of Reference for the SACs must be approved by the EWG.
3.1. JPAC Board
The JPAC Board is required to:
- Oversee and coordinate the work of the EWG and the SACs so as to develop, maintain, produce and communicate the JPAC guidelines.
- Discuss and endorse the guidance produced by the SACs or their subcommittees.
- Ensure that all aspects relevant to the safety of blood, tissues and haemopoietic stem cells are covered by the various SACs and subcommittees.
- Ensure that appropriate discussions are held by the EWG and the SACs, and that the guidelines produced are based on robust evidence wherever possible.
- Ensure appropriate lay representation is included on the JPAC Board and provide clear guidance with respect to the role that they are requested to fulfil within JPAC’s remit.
- Ensure alignment with the legislative environment in the UK, in particular that of the UK Blood Safety and Quality Regulations (BSQR), and with applicable European legislation, such as the guidelines produced by the European Directorate for the Quality of Medicines & HealthCare (EDQM) of the Council of Europe (CoE).
- Ensure the timely updating of guidelines so they always reflect current best practice in the UK.
- Suggest areas for further exploration to appropriate bodies.
- Maintain close collaboration with its stakeholders.
- Support the Professional Director in the preparation of reports and annual workplans for submission to the UK Forum.
While JPAC is not responsible for implementation of guidelines, it is important that JPAC is kept informed of implementation arrangements and dates within the individual UK Services. This is done through engagement with Quality Assurance Managers within each Service.
3.2. Executive Working Group
The EWG is required to:
- Coordinate the workplans of the individual SACs.
- Provide a forum for collaborative discussion between SACs.
- Discuss and refine guideline recommendations presented by SAC Chairs, for subsequent submission to the JPAC Board where further discussion is required.
- Approve guideline changes that do not require consideration or approval by the JPAC Board, as appropriate under the Delegated Approval process in JPAC Ways of Working.
- Conduct regular horizon scanning.
3.3. Standing Advisory Committees
3.3.1. General to all SACs
All SACs and SAC subcommittees are required to:
- Provide professional and technical advice to the UK Services, taking account of the Blood Safety and Quality Regulations (2005), the Human Tissue (Quality and Safety for Human Application) Regulations 2007 and future UK legislation affecting the Services.
- Prepare detailed service guidelines for the UK Services, as the Guidelines for the Blood Transfusion and Tissue Transplantation Services in the UK (Red Book) and its constituent Donor Selection Guidelines, including reviewing and updating relevant chapters.
- Provide documented summaries of the evidence and rationale upon which guidelines are based.
- Keep guidelines up to date in the light of developments in scientific and medical knowledge and changes in the regulatory and legislative environment, consistent with current good practice.
- Provide scientific, medical and technical information that may be adapted by the UK Services for the purposes of staff training.
- Ensure that the EWG and the JPAC Board is kept informed of any significant developments both nationally and internationally within the remit of the SAC.
- Coordinate with other SACs and other relevant UK working groups, where appropriate.
- Contribute content to the JPAC website, where appropriate.
3.3.2. SAC on Blood Components (SACBC)
SACBC is required to:
- Set evidence-based (where possible) specifications for blood components.
- Develop and review validation processes for novel blood components.
- Assess acceptability for use of novel blood components.
- Assess and set requirements for storage and transport systems for blood components.
- Coordinate with SACIT regarding labelling and unique identification of blood components.
- Develop generic protocols for evaluating methods for the collection and processing of blood and blood components.
3.3.3. SAC on Care and Selection of Donors (SACCSD)
SACCSD is required to:
- Review and update guidance on staffing, environment, equipment and procedures for blood donation sessions.
- Coordinate with SACCTP and SACT regarding donor selection criteria.
- Coordinate with SACTTI to ensure integrated advice on all aspects of microbiological safety of donors and donations.
- Be responsible for reviewing and updating the Donor Selection Guidelines for:
- whole blood and components (WB-DSG).
- the Geographical Disease Risk Index (GDRI).
3.3.4. SAC on Cellular Therapy Products (SACCTP)
SACCTP is required to:
- Provide high quality professional advice on matters relating to cellular therapy products, including:
- the assessment, counselling, consent and care of donors or relatives.
- the collection of cells and their processing, quality assessment, storage, transportation and issue for clinical use or other purposes.
- the labelling and unique identification of cellular products.
- the donation of mononuclear cells for advanced therapy medicinal products (ATMP) starter materials.
- Coordinate with SACCSD and SACT regarding donor selection criteria.
- Coordinate with SACTTI to ensure integrated advice on all aspects of microbiological safety of cellular therapy products.
- Be responsible for reviewing and updating the Donor Selection Guidelines for:
- bone marrow and peripheral blood stem cells (BM-DSG).
- cord blood (CB-DSG).
3.3.5. SAC on Immunohaematology (SACIH)
SACIH is required to:
- Set guidelines on the immunohaematological testing of donors and patients by serological and nucleic acid test (NAT) methods.
- Coordinate with other SACs in areas where there is an immunohaematological interest (e.g. HLA screening assays).
- Liaise with MHRA South Mimms Laboratory (formerly NIBSC) on availability, development and use of standard reference preparations and reagents for immunohaematology, histocompatibility and immunogenetics.
3.3.6. SAC on Information Technology (SACIT)
SACIT is required to:
- Define standards and/or guidance for inclusion in the Red Book, in conjunction with other SACs as needed. These standards and/or guidance will cover all services provided by the UK Services. SACIT will be specifically responsible for the management of the following chapters:
- Specification for the uniform labelling of blood, blood components and blood donor samples
- Specification for the uniform labelling of human tissue products using ISBT 128
- Standards for electronic data interchange within the UK Blood Transfusion Services
- Specification for blood pack base labels
- Specification for labelling consumables used in therapeutic product production
- Define information, interoperability and technical standards to support traceability of blood components and associated equipment.
- Ensure appropriate arrangements are in place to administer the UK Services’ database on blood component labels and barcodes.
- Establish and maintain a formal relationship with the International Council for Commonality in Blood Banking Automation (ICCBBA), providing a single point of contact, to consider how changes to ICCBBA standards and requirements need to be adopted in the UK Services and hospitals.
- Recommend, support and advise on (but not to implement) strategies and change programmes for the implementation of standards in the UK Services and hospitals. This includes the establishment of agreed timelines for the implementation of new/changed standards.
- Review relevant international and NHS guidelines, legislation and developments in IT and assess their relevance for the UK.
- Promote commonality and define and recommend standards for the UK Services to JPAC. This will include standards on data structures, delivery mechanisms and labelling.
- Maintain relationships with national and international groups to better understand the development of standards in IT. This will include Blood Products, Tissue Services and Stem Cell standards groups.
3.3.7. SAC on Tissues (SACT)
SACT is required to:
- Provide high quality professional advice on matters relating to tissues from deceased and living donors, including:
- the assessment, counselling, consent and care of donors or relatives.
- the collection of tissues and their processing, quality assessment, storage, transportation and issue for clinical use or other purposes.
- the labelling and unique identification of tissues.
- Coordinate with SACCSD and SACCTP regarding donor selection criteria.
- Coordinate with SACTTI to ensure integrated advice on all aspects of microbiological safety of tissues.
- Be responsible for reviewing and updating the Donor Selection Guidelines for:
- deceased donors of tissue (TD-DSG).
- living donors of tissue (TL-DSG).
3.3.8. SAC on Transfusion Transmitted Infection (SACTTI)
SACTTI is required to:
- Maintain awareness of new or previously unrecognised (‘emerging’) microbiological threats to safety of blood, tissues and stem cells.
- Advise on the epidemiological basis for deferral of particular donor demographic groups, in respect of both recognised and emerging transfusion transmissible agents.
- Recommend laboratory and related procedures for detection and exclusion of donations that may pose a microbiological risk.
- Coordinate with SACCSD and, where appropriate, prepare joint recommendations to JPAC that take account of all relevant aspects of microbiological safety of donors and donations.
- Coordinate with SACBC, SACCTP and SACT on guidance to improve microbiological safety of donations.
- Liaise with MHRA South Mimms (formerly NIBSC) on the availability, development and use of standard reference preparations and reagents for microbiological testing of donors and donations.